[Webinar Recap] Bridging Innovation and Access: A Global Dialogue on Medical Device Reimbursement and Regulatory Pathways
As medical device innovation accelerates globally, mastering the complex interplay of reimbursement and regulation has become essential for turning breakthroughs into accessible healthcare solutions.
As the global healthcare landscape accelerates toward precision medicine, digital therapeutics, and AI-driven care, the success of medical innovations depends not only on regulatory clearance but also on the ability to navigate complex reimbursement systems. On June 25, over a hundred participants from around the world joined a special international webinar to unpack the evolving strategies behind market access for innovative medical devices.
Titled “Reimbursement and Regulatory Trends in Innovative Medical Devices,” this session was hosted by the Science & Technology Law Institute of the Institute for Information Industry (III) and co-organized by the Medical Device Innovation Center at National Cheng Kung University. Guided by the Department of Industrial Technology, the MOEA, and executed by Anke Media Corp., the event marked the inaugural session of a global series designed to connect Taiwan’s medtech ecosystem with international experts and policy insights.
Reimbursement Is Not a One-Step Process in the U.S.
Mr. Mark Domyahn, Partner at JD Lymon Group and a leading U.S. reimbursement strategist, shared practical insights on how medtech companies can succeed in the U.S. market. He emphasized that FDA clearance is just the beginning—true market access depends on a strategy built around three interdependent elements:
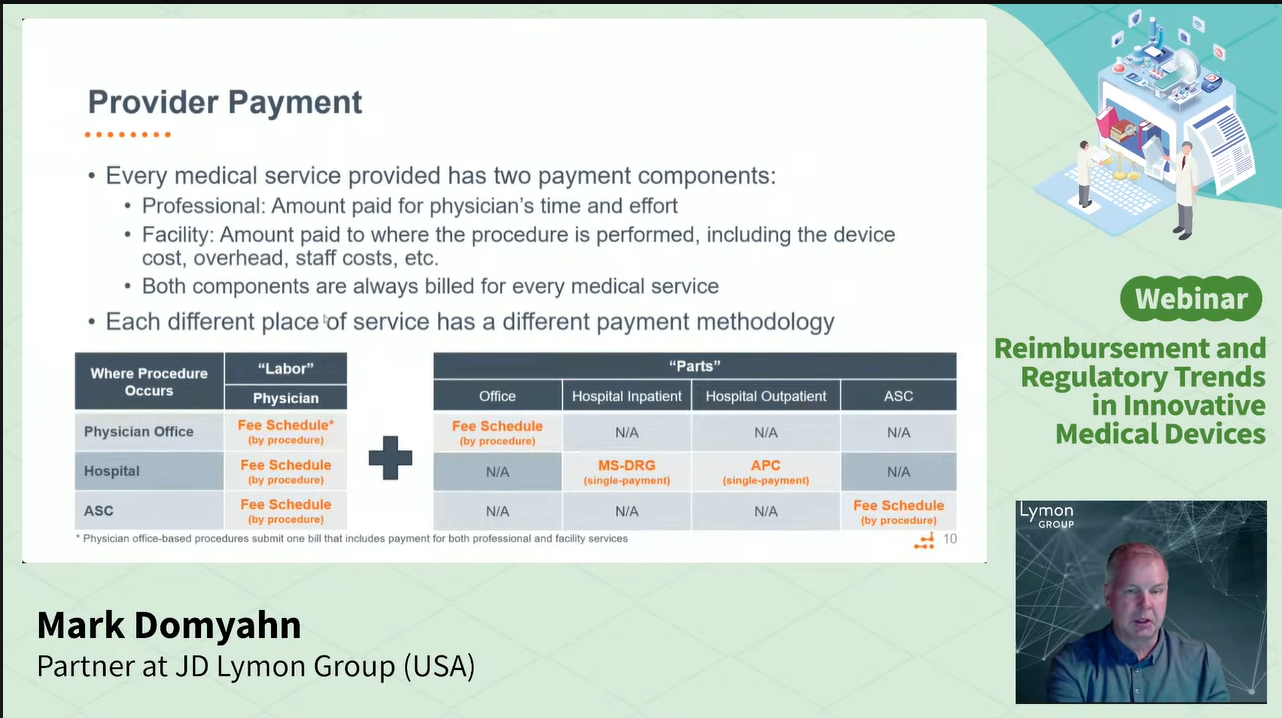
Coding: Defines how a product is identified in billing systems (e.g., CPT, ICD-10, HIPAA). Lack of appropriate codes can significantly delay market entry.
Payment: Determines reimbursement rates, which vary based on care settings (inpatient, outpatient, home, etc.) and product types (e.g., drugs, medical devices, wearable devices).
Coverage: The most critical factor. Even if a product has appropriate coding and favorable reimbursement rates, it may still struggle to enter the market and achieve widespread adoption if payers—such as Medicare or commercial insurers—do not determine that it is medically reasonable and necessary.
“Reimbursement is a journey, not a checklist,” Domyahn said. “Payers are built to say no. Strong clinical evidence is essential.”
He advised early engagement with U.S. stakeholders such as CMS, AMA, and commercial payers, and highlighted the strategic value of programs like Breakthrough Device Designation (BDD), New Technology Add-On Payments (NTAP), and Transitional Pass-Through Payment (TPT) for accelerating market access.
Domyahn also noted that reimbursement models differ for wearables, durable medical equipment (DME), and software-based therapies, often requiring alternative pricing and distribution strategies, particularly for global companies entering the U.S. market.
Building with a Global Mindset Before Going Abroad
Professor Anna Chen of NCKU, also a Stanford Biodesign Global Faculty Fellow, provided a Taiwanese perspective on integrating reimbursement strategy early in product development. She emphasized that Taiwan’s medtech firms must design for global markets from day one.
Observing the rapid increase in FDA De Novo approvals (from 2022 to 2025), she illustrated how non-invasive, AI-driven home-use devices are reshaping the regulatory and reimbursement landscape—especially in fields like neurology and chronic disease.
“Many innovations fail not because of poor technology, but because they enter the ‘valley of death’ without a reimbursement plan,” she warned.
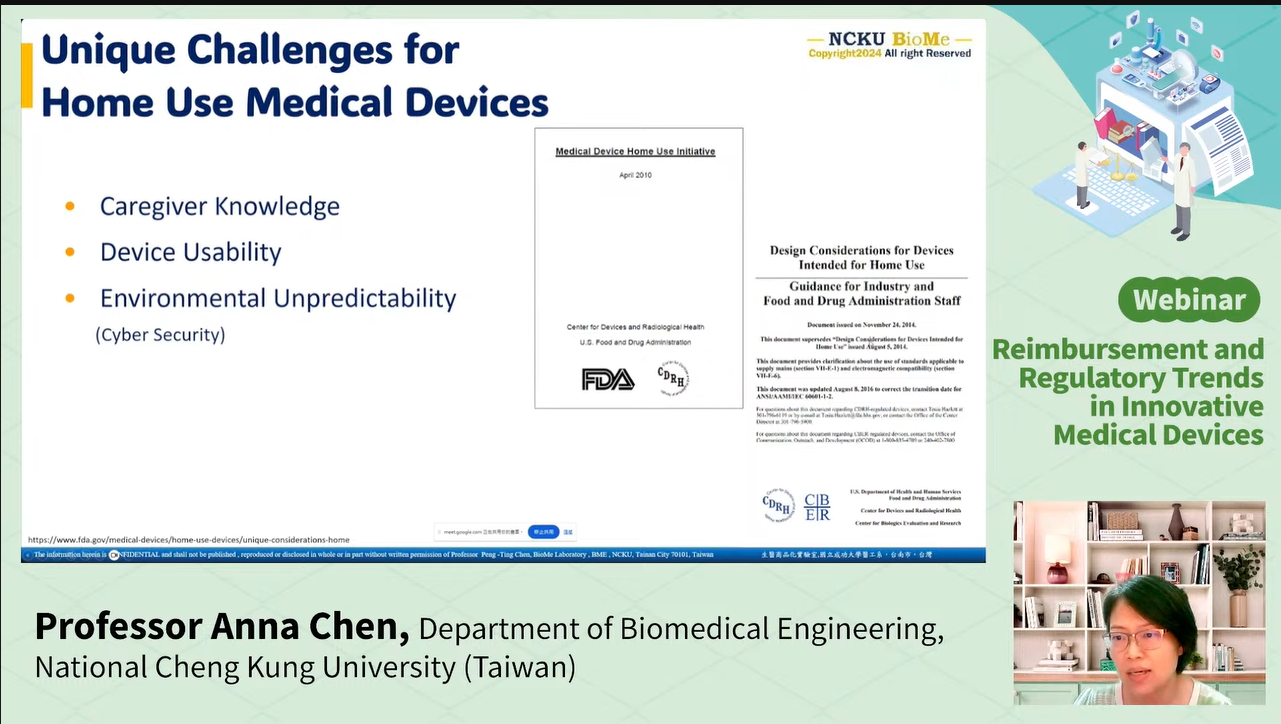
She stressed that clinical studies must generate not only regulatory evidence but also payer-relevant data, such as the outcomes and comparative effectiveness. Chen also discussed challenges unique to home-use and consumer devices, including:
Usability in varied home settings
Cybersecurity and data privacy
Caregiver involvement and training
“Companies should anticipate these issues early, as they’re becoming central to both regulatory approval and market acceptance.”
From Theory to Practice: Questions from Real Innovators
The Q&A session offered valuable insights into the complexities of global market access. Key takeaways included:
On reimbursement planning:
Domyahn emphasized that delaying this consideration can cause significant commercialization setbacks. Professor Chen stressed that for Taiwan’s startups—especially in AI, semiconductors, and software diagnostics—adopting a global-first mindset is critical from the outset.
On pricing strategies for home-use devices:
Domyahn explained that the DME market differs markedly from traditional hospital reimbursement. Companies often need to consider cash-pay or hybrid business models to succeed in this channel.
On preparing for the FDA’s Breakthrough Device Designation (BDD) pathway:
Professor Chen recommended designing clinical study protocols that incorporate standard-of-care comparators, ensuring data robustness to meet regulatory and payer expectations.
On software updates and label expansions:
Impact reimbursement. Domyahn noted that minor software changes typically do not require new codes, but payer coverage may fluctuate depending on how the device’s indications evolve.
On drug versus device reimbursement in the U.S.:
Domyahn clarified that devices are reimbursed based on the associated procedure, not through drug formularies, highlighting the importance of well-published clinical evidence to support payer negotiations.
Webinar Recap: https://www.youtube.com/live/LIUB_oPsmzs
Introduction of the Science & Technology Law Institute of the Institute
Ever since its establishment, the Science & Technology Law Institute (STLI) has been a think tank devoted to the development of science and technology laws and policies and has promoted general industry-wide legal systems. Besides serving as the legal consultant for III, the STLI has used its professional legal expertise to provide practical solutions for the Taiwanese government’s efforts to create a strong legal and regulatory environment for the development of science and technology industries and other emerging industries, and enable these industries to cope with significant challenges emerging from the development of information, technology and globalization. The goal of these efforts has been to enable Taiwan’s industries to keep up with international trends and expand global development.
Official Website: https://stli.iii.org.tw//en/index.aspx
Related Article:[Webinar] Reimbursement & Regulation of Innovative Medical Devices (Registration Now Open / June 25, 2025)