[Webinar Recap] EU Market Access for Software as a Medical Device (SaMD) Decoded: From MDR/IVDR to the AI Act for SaMD regulation and reimbursement
Unlocking EU market access for Software as a Medical Device (SaMD) requires mastering regulatory strategy, reimbursement pathways, and global compliance insights.
The rapid progress of Personalized Precision Medicine, Bio-ICT, and Biomedical Chip Technology is transforming the global MedTech sector. To address the regulatory complexities resulting from the stringent European Union framework, the Science & Technology Law Institute (STLI) of the Institute for Information Industry (III) hosted an international webinar on Reimbursement and Regulatory Trends in Software as a Medical Device (SaMD) in Europe.
Faced with the complexities of the European market and high-risk AI systems, the experts underscored the successful market access for SaMD requires not only compliance with the MDR/IVDR regulation but also alignment with the EU AI Act, as well as integration into QMS and the technical documentation. CE marking under MDR/IVDR does not exempt a high-risk AI system from AI Act compliance. However, The EU AI Act does not replace MDR/IVDR; Manufactures must prepare separate documentation for AI Act conformity. In addition, the AI systems need to comply with GDPR and ensure that they are developed and deployed in a way that respects privacy and ethical standards.
The experts noted that the EU AI Act introduces an additional critical governance layer, thereby enabling the safe and responsible implementation of medical AI, preventing potential harm to EU citizens, and supporting innovation for the adoption, reimbursement, and sustainable commercialization of medical AI.
Keynote Insights: Market Access and Regulatory Compliance
● Strategic Market Access
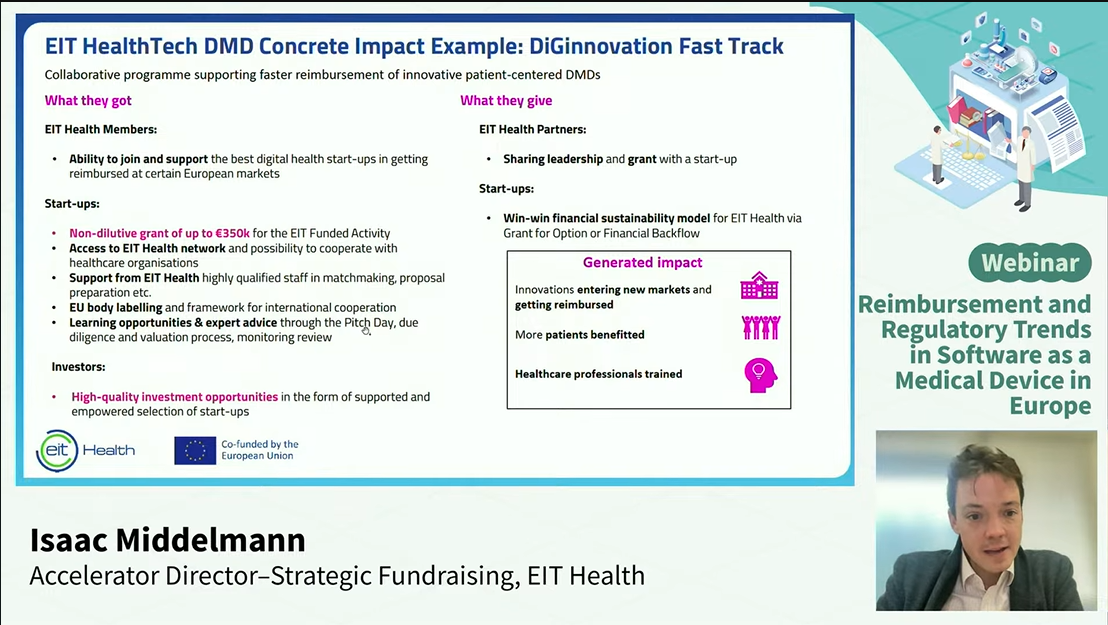
Mr. Isaac Middelman, EIT Health, highlighted key challenges in digital health adoption and payer reimbursement for SaMD. Many healthcare professionals remain hesitant to use digital solutions due to limited understanding or trust, while companies face difficulties in translating SaMD benefits into insurer-accepted clinical endpoints. Additionally, even clinically effective applications may face reimbursement rejection if economic viability is not clearly demonstrated.
He also emphasized the implications of the EU AI Act, which adds a high-risk regulatory layer, and requires compatibility with MDR, IVDR, and GDPR. Non-European companies are advised to identify smaller, more innovative markets for initial entry before scaling, leveraging early-stage collaborations and evidence generation to support regulatory approval and reimbursement.
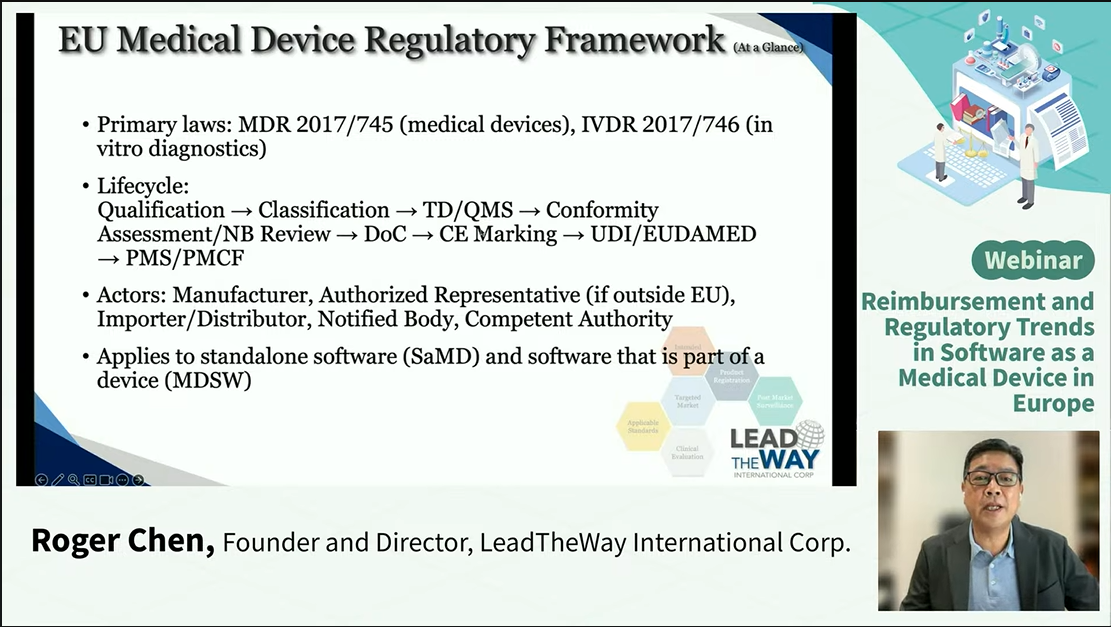
● Technical and safety conformity assessment routes
Mr. Roger Chen, LeadTheWay International Corp., outlined the EU regulatory framework for SaMD and the EU AI Act. Software is classified as SaMD if it performs a medical device function on its own. However, if the software drives/affects the use of a device, it is classified as MDSW (Software that is part of a device). SaMD and MDSW are both subject to risk-based classification, with Notified Body involvement where required, and their conformity assessment routes vary depending on whether the software is integrated with hardware or functions as a standalone product. Conformity assessment requires adherence to recognized quality control, risk assessment, and a full software lifecycle to ensure software’s safety and effectiveness.
The AI Act complements existing MDR/IVDR regulations rather than replacing them. Manufacturers must incorporate additional requirements for AI governance, data management, transparency, and post-market monitoring into their existing quality management systems. Early consultation with Notified Bodies is strongly recommended to ensure smooth regulatory submission and market access.
Q&A Highlights: Strategic Takeaways and Future Outlook
During the Q&A session, the experts discussed key considerations for navigating the converging MDR, IVDR, and AI Act frameworks:
Unified Compliance Approach: Regulatory approval and Health Technology Assessment (HTA) should be treated as a single, integrated process. Early collaboration between R&D and regulatory teams ensures that innovations meet both legal and economic evidence requirements.
Operational Challenges of the EU AI Act: Enforcement remains complex as national authorities in EU member states are still interpreting guidelines. Strong local partnerships with regulatory bodies are recommended to mitigate delays.
Future of HTA: While the EU promotes Joint Clinical Assessments (JCAs) to produce shared EU-level clinical assessments, final reimbursement decisions remain fragmented at the national level, posing an ongoing challenge to market access.
Webinar Recap: https://www.youtube.com/watch?v=e_dsN0LUgTw&t=10s
Webinar Overview: https://reurl.cc/ORqvx9
Introduction of the Science & Technology Law Institute of the Institute
Ever since its establishment, the Science & Technology Law Institute (STLI) has been a think tank devoted to the development of science and technology laws and policies and has promoted general industry-wide legal systems. Besides serving as the legal consultant for III, the STLI has used its professional legal expertise to provide practical solutions for the Taiwanese government’s efforts to create a strong legal and regulatory environment for the development of science and technology industries and other emerging industries, and enable these industries to cope with significant challenges emerging from the development of information, technology and globalization. The goal of these efforts has been to enable Taiwan’s industries to keep up with international trends and expand global development.
Official Website: https://stli.iii.org.tw//en/index.aspx